The Ion product
of water:
In the study of acid-base reactions, the hydrogen ion concentration
is key; its value indicates the acidity or basicity of the solution. Because
only a very small fraction of water molecules are ionized, the concentration of
water, [H2O], remains virtually unchanged (concentration of pure
water, [H2O] = 55.5M). Therefore, the equilibrium constant for the
auto-ionization of water, according to Equation is:
Because we use H+(aq) and H3O+(aq)
interchangeably to represent the hydrated proton, the equilibrium constant can
also be expressed as:
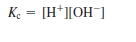
To indicate that the equilibrium constant refers to the
auto-ionization of water, we replace Kc by Kw:

Where Kw is called the ion-product constant, which is
the product of the molar concentrations of H+ and OH-
ions at a particular temperature:
In pure water at 25°C, the concentrations of H+ and OH-
ions are equal and found to be [H+] =1.0x1027 M and
[OH]= 1.0x1027M. Thus, at 25°C:
Whether we have pure water or an aqueous solution of dissolved
species, the following relation always holds at 25°C:
Whenever [H+]=[OH-], the aqueous solution is
said to be neutral. In an acidic solution there is an excess of H+
ions and [H+]>[OH-]. In a basic solution there is an
excess of hydroxide ions, so [H+]<[OH-]. In practice
we can change the concentration of either H+ or OH- ions in
solution, but we cannot vary both of them independently. If we adjust the
solution so that [H+]=1.0x10-6 M, the OH-
concentration must change to:




No comments:
Post a Comment